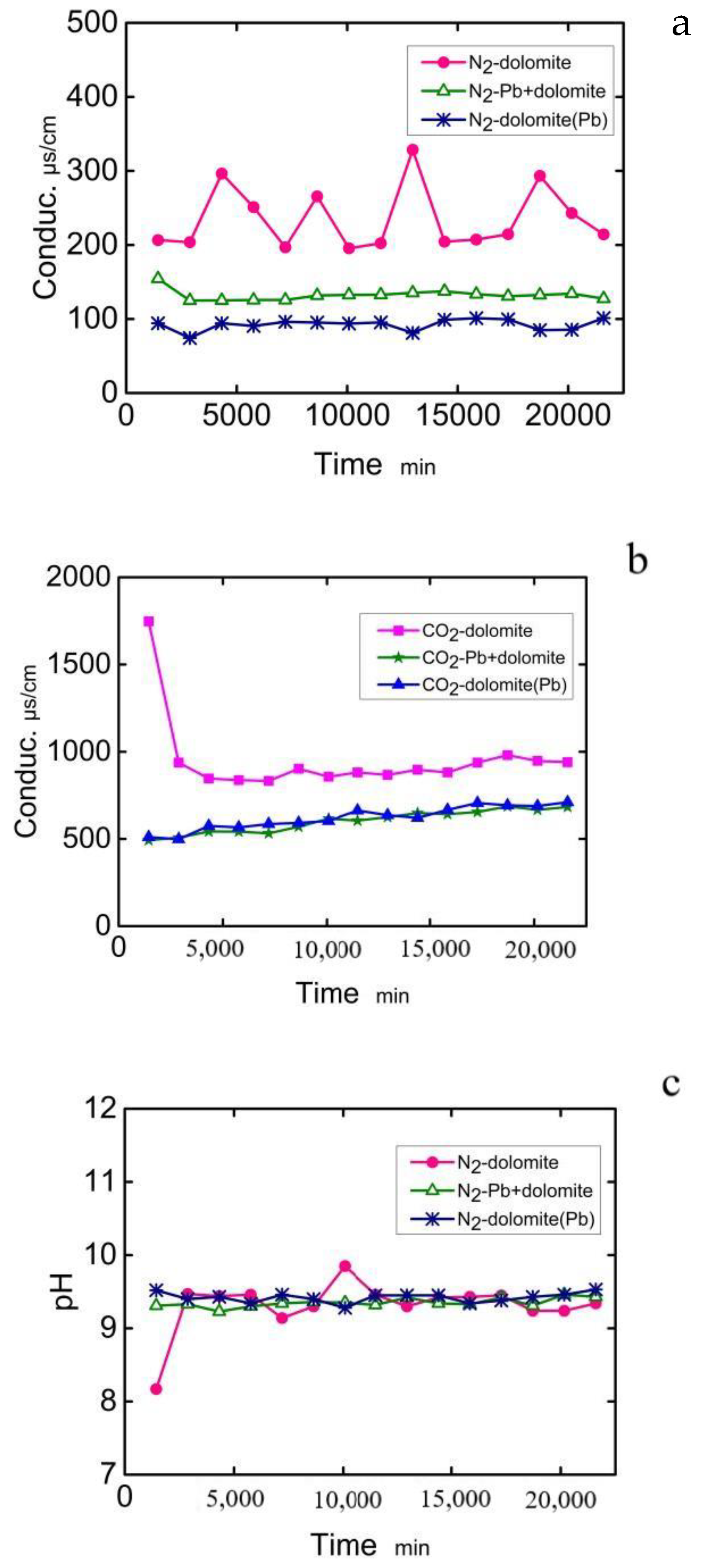
Water | Free Full-Text | Divalent Lead in Aqueous Solution Changes the Surface Morphology of Dolomite and Inhibits Dissolution | HTML
Lecture 9 Chemical Equilibrium and Speciation (1) Supersaturated solutions: (a) have more dissolved substances than predicted b

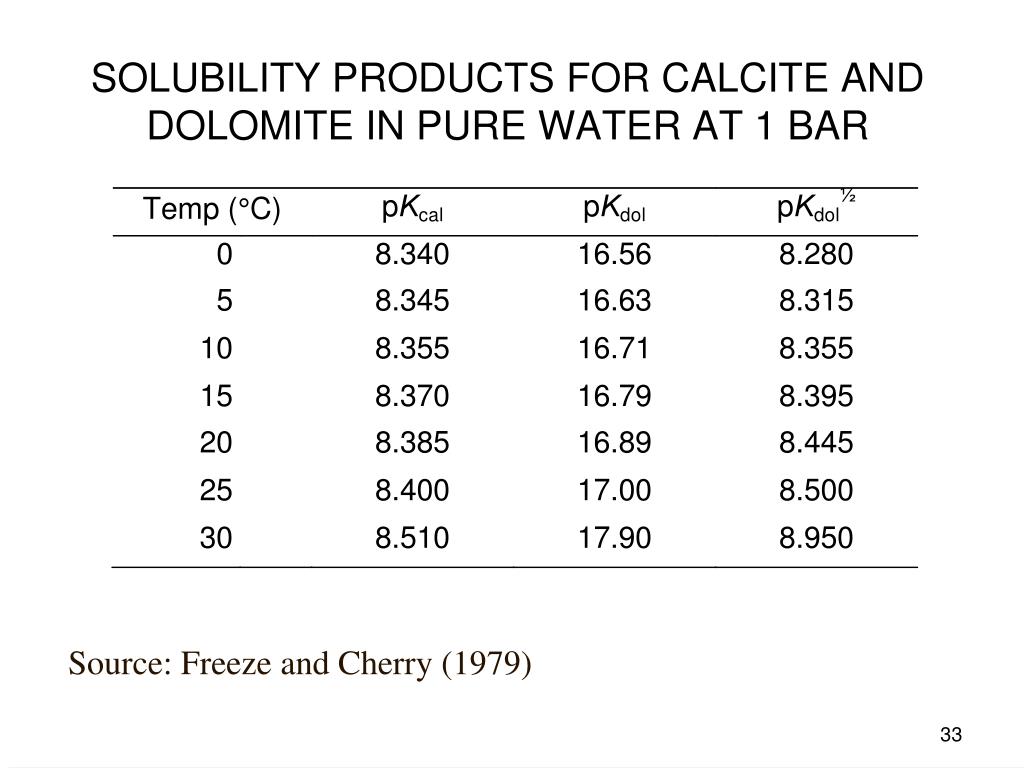
Solubility product constants for natural dolomite (0–200 °C) through a groundwater-based approach using the USGS produced water database | American Journal of Science

Solubility product constants for natural dolomite (0–200 °C) through a groundwater-based approach using the USGS produced water database | American Journal of Science

SE - Precipitation of dolomite from seawater on a Carnian coastal plain ( Dolomites, northern Italy): evidence from carbonate petrography and Sr isotopes

Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library

PDF) Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO/CO Solutions at 25°C and 0.1 MPa Carbon Dioxide

Calcite, dolomite and magnesite dissolution kinetics in aqueous solutions at acid to circumneutral pH, 25 to 150 °C and 1 to 55 atm pCO2: New constraints on CO2 sequestration in sedimentary basins - ScienceDirect
![The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram](https://www.researchgate.net/profile/James-Altland/publication/309875485/figure/fig1/AS:537898228826112@1505256339484/The-solubility-S-of-dolomite-CaMgCO-3-2-as-a-function-of-pH-LlogH-D_Q640.jpg)
The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram

Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library
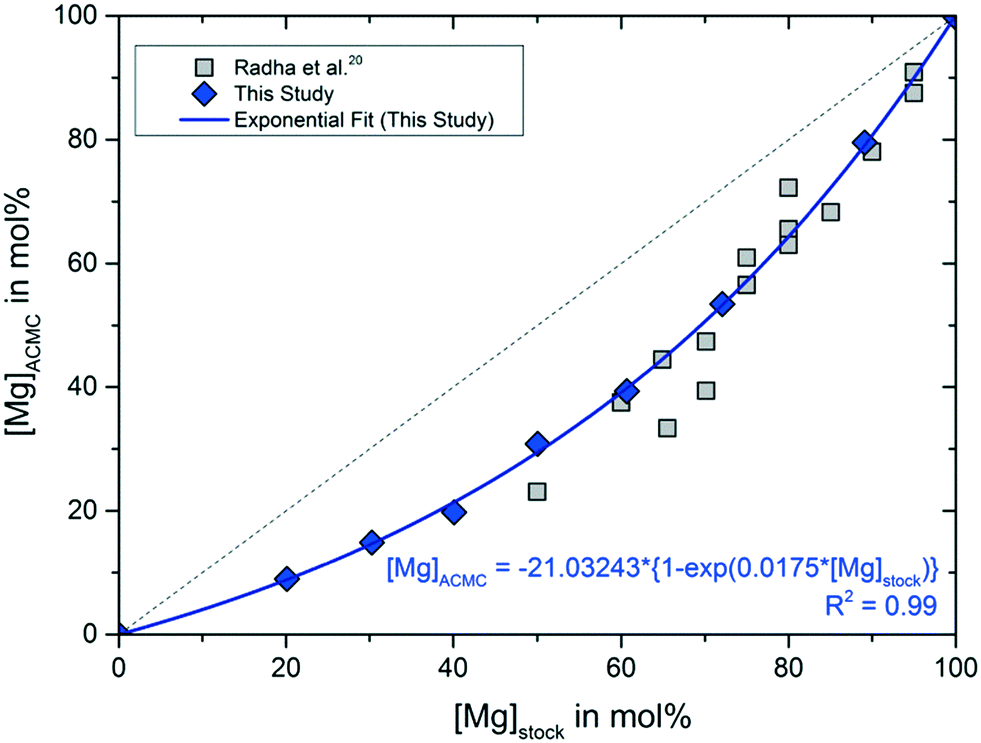
Solubility investigations in the amorphous calcium magnesium carbonate system - CrystEngComm (RSC Publishing) DOI:10.1039/C8CE01596A

Saturation state of calcite and dolomite as a function of pH in the... | Download Scientific Diagram
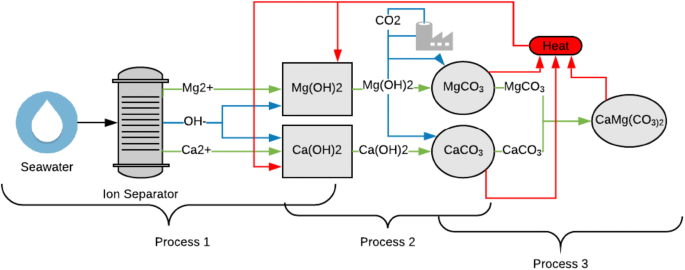
Thermodynamic analysis of theoretical dolomite formation from seawater and captured carbon dioxide | SpringerLink

The impact of Mg2+ ions on equilibration of Mg-Ca carbonates in groundwater and brines - ScienceDirect